What Are N Valence Electrons and Why They Matter in Simple Words
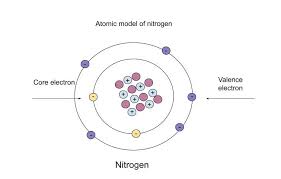
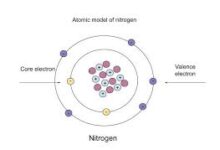
n valence electrons are the outermost electrons found in an atom’s shell. These special electrons help atoms make friends with other atoms, which is called bonding. Without n valence electrons, atoms wouldn’t be able to join together to form the things we see every day like water, air, food, or even our toys. If you’re wondering what the “n” means, it just shows the energy level or shell number where the valence electrons are. It’s a fun and simple way to understand how atoms work. These electrons sit in the last shell and decide what an atom can or cannot do with other atoms. So, knowing about n valence electrons helps us learn how matter is made and why things stick together.
What Are N Valence Electrons? A Super Simple Explanation
n valence electrons are the tiny parts of an atom that live in the outermost shell. They help the atom decide what to do—like whether to join hands with other atoms or stay alone. The “n” shows which shell or energy level the electrons are in. These electrons are the ones that do the bonding magic! Just like friends hold hands to make a circle, atoms use valence electrons to form groups or molecules. If an atom has a full outer shell, it’s happy and doesn’t want to share. But if it’s missing some, it will try to bond with others to fill it. So, understanding n valence electrons helps us know how atoms connect and make everything around us—like water, air, and even food! It may sound small, but these little electrons do some really big work in science.
Why N Valence Electrons Are Important for Every Atom
n valence electrons are super important because they tell us how an atom will behave. Atoms want to feel “complete,” and that happens when their outer shell is full. If it’s not full, the atom will try to bond with another atom to fix that. The number of n valence electrons decides if the atom will share, give, or take electrons during bonding. For example, oxygen has 6 valence electrons, so it needs 2 more to feel happy. So it bonds with atoms that can help it reach 8. This is why water (H2O) is made! Hydrogen gives oxygen those missing electrons. Without these outer electrons, atoms wouldn’t know how to make molecules or compounds. That means no air to breathe, no water to drink, or food to eat. That’s why n valence electrons are not just tiny—they are powerful too!
How to Find N Valence Electrons Using the Periodic Table
Finding n valence electrons is super easy if you know how to look at the periodic table. Just find the element and check its group number. Elements in Group 1 have 1 valence electron. Group 2 has 2. Then Groups 13 to 18 also follow a pattern—just look at the last number of the group to know how many valence electrons there are. For example, carbon is in Group 14, so it has 4 valence electrons. Nitrogen is in Group 15, so it has 5. This trick works great for main group elements. The “n” in n valence electrons stands for the shell or energy level the electrons are in. The row number of the periodic table also helps you know which shell they’re sitting in. So just like reading a map, you can use rows and columns to find these important electrons and understand any atom better.
Fun Examples of N Valence Electrons in Everyday Life
You might not see n valence electrons, but they’re behind so many things around you. Take salt for example—when sodium (Na) gives away its one valence electron to chlorine (Cl), they become best friends and form table salt. In water, hydrogen and oxygen share valence electrons to form H2O. Even the air we breathe has oxygen and nitrogen atoms that bond using valence electrons. Without these special electrons, we wouldn’t have soap, plastic toys, or even the yummy food we eat. When we cook, clean, or play, we’re using things that were created through bonding, all thanks to n valence electrons. So the next time you sip water or bite a sandwich, remember: those tiny electrons in the outer shell made it happen. That’s why they are so cool and helpful in real life—always busy, always bonding!
What N Valence Electrons Tell Us About an Atom’s Personality
Just like people have personalities, atoms do too—and n valence electrons tell us what kind of “person” the atom is. Atoms with almost full valence shells, like chlorine or oxygen, are eager to grab more electrons. They’re like kids who want more crayons to finish coloring. Atoms with just one or two valence electrons, like sodium or potassium, want to give theirs away so they can feel complete. They’re like friends who love sharing! If an atom has a full shell, like neon or helium, it doesn’t want to bond at all—it’s already happy and chill. So by looking at the number of valence electrons, we can tell if an atom is a giver, a taker, or just wants to be left alone. These little electrons help us understand what the atom wants to do, and that makes science super fun and easy!
Conclusion
Atoms may be small, but they are full of surprises. Thanks to n valence electrons, we can understand how atoms act and how they team up to make the world around us. Without them, there would be no bonding, no molecules, and no fun experiments! These little electrons are like the glue that holds everything together.
So the next time you see water, food, or even a toy, think about those hardworking n valence electrons behind the scenes. They may be tiny, but they do huge jobs every day. And now that you know about them, science will make a lot more sense and feel a lot more exciting!
FAQs
Q: What are n valence electrons?
A: They are the outermost electrons in an atom’s shell that help atoms bond with other atoms.
Q: Why are n valence electrons important?
A: They help atoms make bonds and create molecules like water, salt, and more.
Q: How do I find n valence electrons in an element?
A: Use the periodic table and check the group number of the element.
Q: Do all atoms have n valence electrons?
A: Yes, all atoms have valence electrons, and “n” shows which shell they’re in.
Q: Can n valence electrons change?
A: No, each element has a fixed number of valence electrons unless it forms ions.